-
Fil d’actualités
- EXPLORER
-
Pages
-
Groupes
-
Blogs
-
Développeurs
Automatic Power Factor Controller Market at a Glance: Size, Growth, and Challenges Ahead
"Executive Summary Automatic Power Factor Controller Market Size and Share: Global Industry Snapshot
Global automatic power factor controller market was valued at USD 5013.52 million in 2021 and is expected to reach USD 7129.73 million by 2029, registering a CAGR of 4.50% during the forecast period of 2022-2029.
Automatic Power Factor Controller Market report offers the most appropriate solution for the business requirements in many ways. To be successful in this competitive age, it is very imperative to get well-versed about the major happenings in the Automatic Power Factor Controller Market industry which is possible only with the excellent market report like this one. To make aware about the industry insights so that business never misses anything, this is the valuable market report. The report also analyzes the market status, market share, growth rate, sales volume, future trends, market drivers, market restraints, revenue generation, opportunities and challenges, risks and entry barriers, sales channels, and distributors. A large scale Automatic Power Factor Controller Market report not only assists with the informed decision making but also helps with smart working.
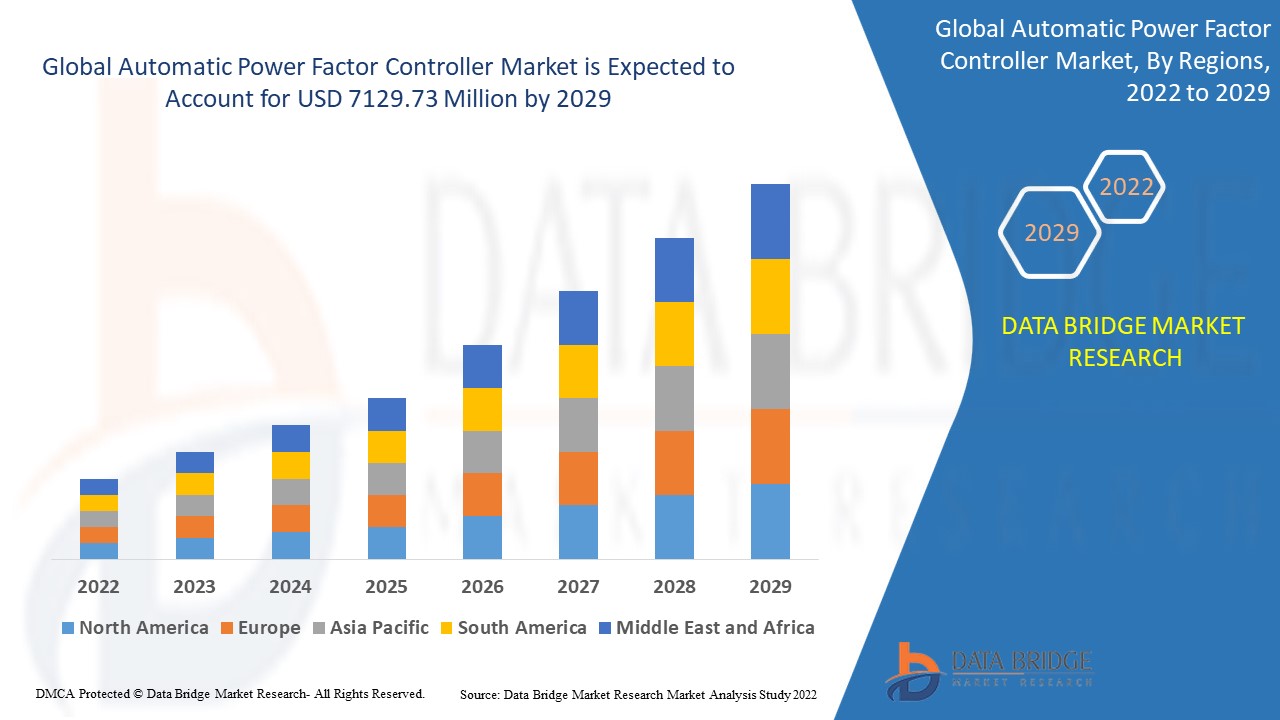 The top notch Automatic Power Factor Controller Market report defines various segments related to Automatic Power Factor Controller Market industry and market with thorough research and analysis. These can be listed as; industry outlook, critical success factors (CSFs), industry dynamics, market drivers, market restraints, market segmentation, value chain analysis, key opportunities, application and technology outlook, regional or geographical insight, country-level analysis, key company profiles, competitive landscape, and company market share analysis. So, business can surely go with an all-embracing Automatic Power Factor Controller Market research report to take business to the highest level of growth and success.
The top notch Automatic Power Factor Controller Market report defines various segments related to Automatic Power Factor Controller Market industry and market with thorough research and analysis. These can be listed as; industry outlook, critical success factors (CSFs), industry dynamics, market drivers, market restraints, market segmentation, value chain analysis, key opportunities, application and technology outlook, regional or geographical insight, country-level analysis, key company profiles, competitive landscape, and company market share analysis. So, business can surely go with an all-embracing Automatic Power Factor Controller Market research report to take business to the highest level of growth and success.
Stay informed with our latest Automatic Power Factor Controller Market research covering strategies, innovations, and forecasts. Download full report: https://www.databridgemarketresearch.com/reports/global-automatic-power-factor-controller-market
Automatic Power Factor Controller Market Trends & Analysis
**Segments**
- Based on type, the automatic power factor controller market can be segmented into open loop control system and closed loop control system. Open loop control systems are more traditional and operate based on a preset power factor value, whereas closed loop systems continuously monitor the power factor and adjust it in real time.
- On the basis of component, the market can be categorized into capacitors, relays, display units, microcontrollers, and others. Capacitors play a crucial role in improving power factor, while relays help in switching the capacitors on and off as needed.
- By end-user, the automatic power factor controller market can be divided into industrial, commercial, and residential sectors. Industries have a high demand for these controllers to avoid penalties associated with poor power factor, while commercial and residential users are increasingly adopting these systems for energy efficiency purposes.
**Market Players**
- ABB Ltd.
- Schneider Electric
- Eaton
- Siemens
- General Electric
- Mitsubishi Electric Corporation
- Larsen & Toubro Limited
- Crompton Greaves
- EPCOS AG
- Littelfuse Inc.
The automatic power factor controller market is expected to witness significant growth in the coming years due to the increasing focus on energy efficiency and the growing demand for electricity in various sectors. Factors such as government regulations promoting the use of power factor correction devices, rising industrialization leading to a surge in power consumption, and the need to reduce electricity bills are driving the market. The adoption of automatic power factor controllers helps in stabilizing voltage levels, improving power quality, and reducing overall energy consumption. Industries, in particular, are investing in these controllers to enhance operational efficiency and minimize losses associated with reactive power.
The key players in the market are continuously focusing on technological advancements and product innovations to gain a competitive edge. Partnerships, collaborations, and acquisitions are also common strategies employed by these companies to expand their market presence and enhance their product portfolio. The market players mentioned above have a strong global presence and offer a wide range of automatic power factor controllers to cater to the diverse needs of end-users across different industries.
Overall, the automatic power factor controller market is poised for robust growth driven by the increasing emphasis on energy conservation and the rising awareness about the benefits of power factor correction devices. With advancements in technology and the implementation of favorable government policies, the market is expected to witness steady expansion in the forecast period.
The automatic power factor controller market is undergoing a transformation driven by the increasing need for energy efficiency and the growing demand for electricity across various sectors globally. One of the emerging trends in the market is the integration of smart technologies in power factor controllers, enabling real-time monitoring and adjustment of power factor values. This trend is gaining traction as companies strive to optimize their energy consumption and reduce operational costs. Furthermore, the rising awareness about the detrimental effects of poor power factor on electrical systems is compelling industries to invest in advanced power factor correction solutions.
Another significant development in the market is the emphasis on sustainability and environmental conservation. Automatic power factor controllers play a vital role in reducing carbon emissions and minimizing energy wastage, aligning with the sustainability goals of many organizations. Additionally, the shift towards renewable energy sources has created new opportunities for the market players to develop innovative solutions that can effectively manage power factors in hybrid energy systems.
Moreover, the increasing application of automatic power factor controllers in data centers, healthcare facilities, and commercial buildings is reshaping the market dynamics. These sectors require stable power supply and optimal power factor levels to ensure smooth operations and minimize downtime. As a result, there is a burgeoning demand for customized power factor correction solutions that can address the specific requirements of different end-users.
Furthermore, advancements in IoT and cloud-based technologies are revolutionizing the automatic power factor controller market by enabling remote monitoring and predictive maintenance capabilities. These digital solutions enhance operational efficiency, reduce maintenance costs, and extend the lifespan of power factor correction equipment. Market players are leveraging these technologies to offer integrated solutions that provide comprehensive monitoring and control functionalities to end-users.
In conclusion, the automatic power factor controller market is witnessing a significant shift towards smart, sustainable, and technologically advanced solutions. The focus on energy conservation, coupled with regulatory support and increasing awareness about the benefits of power factor correction devices, is driving market growth. As companies continue to invest in power quality improvement measures and automation technologies, the market is poised for substantial expansion in the foreseeable future. The key to success for market players lies in innovation, strategic partnerships, and a customer-centric approach to meet the evolving needs of a diverse range of industries.The automatic power factor controller market is experiencing a notable transformation propelled by the rising global demand for electricity and the increasing emphasis on energy efficiency across various sectors. The integration of smart technologies into power factor controllers is a key trend reshaping the market dynamics. This integration enables real-time monitoring and adjustment of power factor values, allowing companies to optimize energy consumption and reduce operational costs effectively. Additionally, there is a significant shift towards sustainability and environmental conservation, with automatic power factor controllers playing a crucial role in reducing carbon emissions and minimizing energy wastage, aligning with the sustainability goals of organizations worldwide.
One of the emerging opportunities in the automatic power factor controller market is the increasing application of these systems in data centers, healthcare facilities, and commercial buildings. These sectors require stable power supply and optimal power factor levels to ensure uninterrupted operations and minimize downtime, driving the demand for customized power factor correction solutions tailored to meet specific end-user requirements. Furthermore, advancements in IoT and cloud-based technologies are revolutionizing the market by enabling remote monitoring and predictive maintenance capabilities. These digital solutions not only enhance operational efficiency but also reduce maintenance costs and prolong the lifespan of power factor correction equipment.
As the market continues to evolve, market players are focusing on innovation, strategic partnerships, and a customer-centric approach to address the evolving needs of a diverse range of industries. The emphasis on energy conservation, coupled with regulatory support and increasing awareness about the benefits of power factor correction devices, is fueling market growth. Companies are investing in power quality improvement measures and automation technologies to meet the growing demands for efficient energy management solutions. With the ongoing advancements in technology and the adoption of sustainable practices, the automatic power factor controller market is poised for substantial expansion in the foreseeable future, offering new opportunities for players to leverage smart, sustainable, and technologically advanced solutions to drive market growth and meet the changing needs of the industry.
Learn about the company’s position within the industry
https://www.databridgemarketresearch.com/reports/global-automatic-power-factor-controller-market/companies
Automatic Power Factor Controller Market Overview: Strategic Questions for Analysis
- What does the current research say about the size of the Automatic Power Factor Controller Market?
- What is the predicted CAGR until the end of the forecast period?
- What are the significant components of the Automatic Power Factor Controller Market segmentation?
- Which market players hold a competitive edge?
- What innovations have taken place recently in the Automatic Power Factor Controller Market?
- What countries form the scope of the geographical study?
- Which region holds the title of fastest-growing?
- Which country is expected to hold a leadership position?
- Where is the majority of Automatic Power Factor Controller Market value concentrated?
- Which country’s growth outpaces others?
Browse More Reports:
Global Thermoplastic Polyurethane (TPU) Films Market
Global Thin Wall Plastic Container Market
Global Thrombin Time Testing Market
Global Thyristors Market
Global Toilet Seats Market
Global Touchscreen Controller Market
Global Trace Mineral Supplements Market
Global Trailer Assist System Market
Global Transparent Cache Market
Global Transparent Food Packaging Market
Global Transportation and Logistics Carbon Management System Market
Global Tricyclic antidepressants Market
Global Trimethyl Pentanediol Monoisobutyrate Market
Global Tunable filter Market
Global Two-Factor Authentication Market
Middle East and Africa SWIR Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]
"
- Books
- Software
- Courses
- Film
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
