-
Newsfeed
- ERKUNDEN
-
Seiten
-
Gruppen
-
Blogs
-
Entwickler
In Vivo Toxicology Market Trends: Growth, Share, Value, Size, and Analysis By 2033
Latest Insights on Executive Summary In Vivo Toxicology Market Share and Size
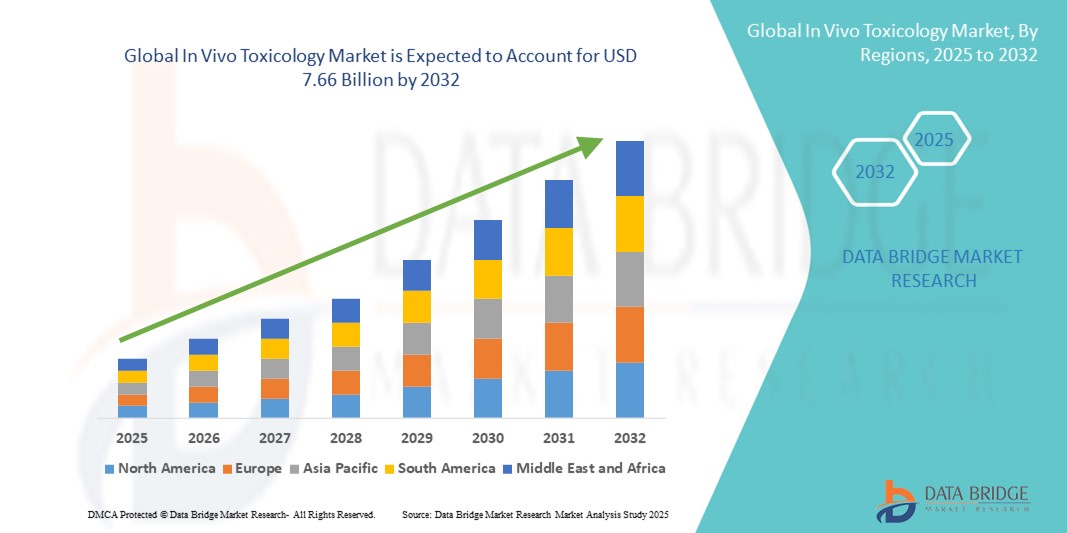
- The global in vivo toxicology market size was valued at USD 4.95 billion in 2024 and is expected to reach USD 7.66 billion by 2032, at a CAGR of 5.60% during the forecast period.
This In Vivo Toxicology Market research report proves to be true in serving the purpose of businesses of making enhanced decisions, deal with marketing of goods or services, and achieve better profitability by prioritizing market goals. This market research report deeply analyses the potential of the market with respect to current scenario and the future prospects by taking into view numerous industry aspects. The In Vivo Toxicology Market report explains market definition, currency and pricing, market segmentation, market overview, premium insights, key insights and company profile of the major market players. Moreover, the In Vivo Toxicology Market report endows with the data and information for actionable, most recent and real-time market insights which make it uncomplicated to take critical business decisions.
The In Vivo Toxicology Market report displays the systematic investigation of existing scenario of the market, which takes into account several market dynamics. The market report also helps to get idea about the types of consumers, their reaction and views about particular products, and their thoughts for the improvement of a product. Geographical scope of the products is also taken into consideration comprehensively for the major global areas which helps characterize strategies for the product distribution in those areas. This In Vivo Toxicology Market research report can be used to acquire valuable market insights in a cost-effective way.
Dive into the future of the In Vivo Toxicology Market with our comprehensive analysis. Download now:
https://www.databridgemarketresearch.com/reports/global-in-vivo-toxicology-market
In Vivo Toxicology Business Outlook
**Segments**
- On the basis of product type, the in vivo toxicology market can be segmented into instruments, reagents & kits, software, and services. The instruments segment includes products such as automated systems, mass spectrometers, and chromatography systems. Reagents & kits consist of assay kits, cell-based assays, and reagents, among others. Software plays a crucial role in data analysis and management for in vivo toxicology studies. Services offered in this market include toxicology testing services, safety assessment, and consultancy services.
- By test type, the market can be categorized into developmental toxicology, genetic toxicity, carcinogenicity testing, and other toxicology tests. Developmental toxicology studies the impact of substances on the growth and development of embryos or fetuses. Genetic toxicity testing assesses the potential of a substance to cause genetic mutations. Carcinogenicity testing evaluates the ability of a substance to induce cancer. Other toxicology tests encompass various endpoints such as neurotoxicity, immunotoxicity, and reproductive toxicity.
- Based on toxicity endpoints, the in vivo toxicology market can be segmented into acute toxicity, sub-acute toxicity, sub-chronic toxicity, and chronic toxicity. Acute toxicity studies focus on the adverse effects of a single exposure to a substance within a short period. Sub-acute toxicity examines the effects of repeated exposure over weeks to months. Sub-chronic toxicity studies extend the exposure duration to a few months. Chronic toxicity assessments span over a significant portion of the subject's lifespan, evaluating prolonged exposure effects of substances.
**Market Players**
- Some of the key players in the global in vivo toxicology market include Charles River Laboratories, Inc., Envigo, Eurofins Scientific, Thermo Fisher Scientific, Inc., Covance Inc., SGS SA, Merck KGaA, and Laboratory Corporation of America Holdings. These companies specialize in providing a range of products and services for in vivo toxicology studies, catering to pharmaceutical companies, biotechnology firms, academic research institutions, and regulatory bodies. With a focus on innovation, strategic collaborations, and expanding service portfolios, these market players aim to enhance their market presence and meet the evolving needs of the industry.
To access more detailed insights on the global in vivo toxicology market, visit .The global in vivo toxicology market is witnessing significant growth due to the increasing focus on ensuring the safety and efficacy of pharmaceuticals and chemicals before they reach the market. With the rising prevalence of chronic diseases and the expanding pharmaceutical and biotechnology industries, the demand for in vivo toxicology studies is poised to escalate. As advancements in technology continue to drive innovation in the sector, market players are emphasizing the development of sophisticated instruments, robust reagents, and efficient software solutions to streamline toxicology testing processes. Additionally, the provision of comprehensive services such as toxicology testing, safety assessment, and consultancy is enabling companies to offer end-to-end solutions to their clients, further propelling market growth.
Segmentation of the in vivo toxicology market based on product type allows for a focused analysis of the diverse offerings within the industry. Instruments such as automated systems, mass spectrometers, and chromatography systems play a crucial role in conducting in vivo toxicology studies with precision and accuracy. Reagents & kits comprising assay kits, cell-based assays, and specialized reagents facilitate specific testing protocols, enhancing the efficiency of toxicology assessments. Software solutions have become integral for data management and analysis in in vivo toxicology, enabling researchers to derive meaningful insights and ensure regulatory compliance. Services including toxicology testing services and safety assessments cater to the outsourcing needs of companies looking to conduct thorough toxicology evaluations.
The categorization of the market by test type provides a nuanced understanding of the different aspects of toxicology studies conducted. Developmental toxicology, genetic toxicity, carcinogenicity testing, and other toxicology tests each address distinct endpoints and potential risks associated with chemical exposure. Developmental toxicology focuses on assessing the impact of substances on embryonic and fetal development, highlighting the importance of early-stage safety evaluations. Genetic toxicity testing helps identify substances that may cause genetic mutations, informing risk assessment and regulatory decision-making. Carcinogenicity testing plays a vital role in evaluating the potential of a substance to induce cancer, guiding the safe development of pharmaceuticals and chemicals. Other toxicology tests covering endpoints like neurotoxicity and reproductive toxicity offer a comprehensive evaluation of substance effects on multiple physiological systems.
Segmenting the market based on toxicity endpoints allows for a detailed analysis of the duration and intensity of substance exposure in toxicology studies. Acute toxicity studies provide insights into the immediate adverse effects of short-term exposure, crucial for determining safe dosages and assessing acute toxicity risks. Sub-acute toxicity assessments extend the exposure duration to evaluate repeated effects over weeks to months, offering a more comprehensive understanding of sub-chronic toxicity effects that span a few months. Chronic toxicity studies, spanning significant portions of subjects' lifespans, offer insights into the long-term effects of prolonged substance exposure, essential for assessing chronic toxicity risks and ensuring product safety.
In conclusion, the global in vivo toxicology market is characterized by a diverse range of products, services, and testing types aimed at ensuring the safety and efficacy of pharmaceuticals and chemicals. Key market players are actively engaged in innovation, strategic partnerships, and expanding their service portfolios to cater to the evolving needs of the industry. With a growing emphasis on regulatory compliance and comprehensive safety assessments, the in vivo toxicology market is poised for continued expansion, driven by technological advancements and increasing demand for reliable toxicology testing solutions.The global in vivo toxicology market is characterized by a robust segmentation based on product types, test types, and toxicity endpoints, reflecting the diverse nature of toxicology studies and the range of services offered by market players. The segmentation by product type highlights the critical role of instruments, reagents & kits, software, and services in conducting in vivo toxicology assessments. Instruments such as mass spectrometers and chromatography systems enable precise data collection, while reagents & kits provide the necessary tools for specific testing protocols. Software solutions streamline data analysis and management, ensuring regulatory compliance and efficient study outcomes. Services offered, including testing, safety assessment, and consultancy, cater to the varied needs of clients seeking comprehensive toxicology solutions.
Moreover, the categorization of the market by test type offers a comprehensive understanding of the different aspects of toxicology evaluations conducted in the industry. Developmental toxicology focuses on assessing the impact of substances on embryonic and fetal development, emphasizing the importance of early-stage safety assessments. Genetic toxicity testing helps identify substances with the potential to cause genetic mutations, informing risk evaluations and regulatory decisions. Carcinogenicity testing plays a crucial role in determining a substance's ability to induce cancer, guiding safe product development practices. Other toxicology tests cover endpoints like neurotoxicity and reproductive toxicity, providing a holistic evaluation of substances on various physiological systems.
Furthermore, segmenting the market based on toxicity endpoints enables a detailed analysis of substance exposure durations and intensities in toxicology studies. Acute toxicity studies offer insights into immediate adverse effects following short-term exposure, aiding in dose determination and acute toxicity risk assessment. Sub-acute toxicity assessments extend exposure durations to evaluate repeated effects over weeks to months, providing a more comprehensive understanding of sub-chronic toxicity effects over a few months. Chronic toxicity studies, spanning significant periods of subjects' lifespans, offer insights into the long-term effects of prolonged exposure, essential for assessing chronic toxicity risks and ensuring product safety.
In conclusion, the segmented nature of the global in vivo toxicology market underscores the depth and breadth of services and solutions offered by key market players to meet the evolving needs of pharmaceutical, biotechnology, and regulatory entities. The emphasis on innovation, strategic collaborations, and service expansion is driving market growth, supported by advancements in technology and increased demand for reliable toxicology testing solutions. Moving forward, the market is poised for continued expansion, driven by regulatory compliance requirements, technological advancements, and the growing focus on comprehensive safety assessments in the pharmaceutical and chemical industries.
Analyze detailed figures on the company’s market share
https://www.databridgemarketresearch.com/reports/global-in-vivo-toxicology-market/companies
In Vivo Toxicology Market – Analyst-Ready Question Batches
- What is the current demand volume of the In Vivo Toxicology Market?
- How is the market for In Vivo Toxicology expected to evolve in the next decade?
- What segmentation criteria are applied in the In Vivo Toxicology Market study?
- Which players have the highest market share in the In Vivo Toxicology Market?
- What regions are assessed in the country-level analysisfor In Vivo Toxicology Market?
- Who are the top-performing companies in the In Vivo Toxicology Market?
Browse More Reports:
Global Fish Meal for Aquaculture Market
Global Fixed Network Lawful Interception Market
Global Flat Glass Market
Global Flea Products Market
Global Flooring Chemical Market
Global Fluid Transfer System Market
Global Food Deaerators for Beverages Application Market
Global Food Grade and Animal Feed Grade Salt Market
Global Food Grade Iron Powder Market
Global Foodservice Disposables Market
Global Food Sterilization Equipment Market
Global Foosball Market
Global Fortified Beverages Market
Global Fractional Distillation System Market
Global Friction Materials Market
Asia-Pacific Microplate Reader Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]
- Books
- Software
- Gruppen
- Filme
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
