-
Noticias Feed
- EXPLORE
-
Páginas
-
Grupos
-
Blogs
-
Desarrolladores
Immuno In-Vitro Diagnostics (IVD) Market Forecast 2025–2032: Global Growth, Innovations, and Key Opportunities in Diagnostic Technology
Introduction
Immuno In-Vitro Diagnostics (IVD) Market refers to diagnostic tests done outside the body (e.g. in laboratory, point-of-care settings) that utilize immunological reactions—such as antigen-antibody binding, immunoassays, rapid tests etc.—to detect, monitor, or manage disease. These tools are vital in detecting infectious diseases, autoimmune disorders, cancer markers, metabolic conditions, and more. As global healthcare systems face twin pressures of rising chronic and infectious disease burdens, aging populations, and demand for early detection and precision medicine, the immuno IVD market is becoming one of the fastest growing diagnostic segments.
Request for Sample PDF For Free: https://www.databridgemarketresearch.com/request-a-sample?dbmr=global-immuno-ivd-market
Market Size & Forecast
-
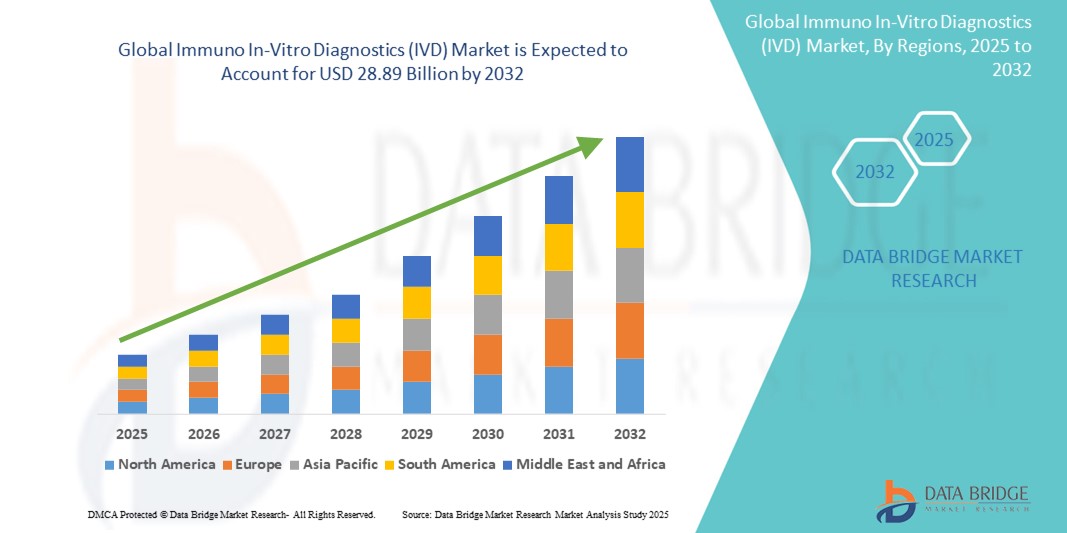
Current Valuation (2024): The global immuno IVD market is estimated to be worth around USD 19.89 billion.
-
Projected Value (2032): It is expected to increase to approximately USD 28.89 billion by 2032.
-
CAGR (2025-2032): The market is forecasted to grow at a compound annual growth rate of about 4.78% over this period.
These figures reflect steady growth—driven by multiple structural factors in healthcare (disease prevalence, diagnostics demand, immunodiagnostic technology innovation, public health initiatives, etc.).
Market Share & Regional Insights
-
Dominant Region: North America holds the largest share of the immuno IVD market, accounting for nearly 49% of total market share. This dominance is due to well-established healthcare infrastructure, high diagnostic test adoption, robust reimbursement systems, regulatory support, and presence of major diagnostic players.
-
Fastest-Growing Region: Asia-Pacific is expected to exhibit the highest growth rate in the forecast period. Contributors include expanding diagnostic infrastructure, rising disease awareness, growing middle class, increased government spending on public health, and rising demand for early detection of infectious and lifestyle-related diseases. Countries like China, India, South Korea, Japan, and Southeast Asian nations are key growth engines.
Market Segmentation & Key Application Insights
To understand where growth is concentrated, here are important segmentation angles:
-
By Product Type:
The immuno IVD market is segmented into Reagents, Instruments, Data Management Software, and Services. Among these, reagents dominate, with a large share of around 66-70% in near term years. This is because reagents are consumables, used repeatedly in immunoassays, rapid tests, etc., and are essential to almost all testing workflows. -
By Technique / Immunodiagnostics Method:
Techniques include Enzyme-Linked Immunosorbent Assay (ELISA), Rapid Tests, Enzyme-Linked ImmunoSpot Assay (ELISPOT), Radioimmunoassay, Western Blotting, etc. Rapid tests and ELISA are especially important in infectious disease detection and in point-of-care settings. -
By Application:
Key application areas include Infectious Diseases, Oncology, Autoimmune Diseases, HIV/AIDS, Diabetes, Cardiology, Nephrology, among others. Of these, infectious diseases often dominate in terms of test volume and public health priority, especially during outbreaks or endemic disease management. Oncology, autoimmune disease diagnostics, and chronic conditions are growing fast as well.
See what’s driving the Europe Automotive Logistics Market forward. Get the full research report: https://www.databridgemarketresearch.com/reports/global-immuno-ivd-market
Key Market Trends
Several trends are shaping the immuno IVD market across technology, application, regulation, and business models:
-
Rise of Rapid & Point-of-Care (POC) Immunodiagnostics:
The demand for rapid, convenient diagnostics that provide quick results outside central labs is increasing. This is especially true for infectious disease screening, emergency settings, and remote or underserved areas. -
Personalized Medicine & Biomarkers:
There is increasing emphasis on using immunodiagnostic tests to detect specific biomarkers for cancer, autoimmune conditions, or therapy monitoring. This supports tailored treatment plans and better outcomes. -
Aging Population & Rising Disease Burden:
Demographics are shifting globally, with more elderly populations who are more susceptible to chronic diseases, cancers, and immune dysfunctions. This magnifies demand for diagnostics for early detection and monitoring. -
Technological Innovation & Automation:
Improved immunoassay techniques (higher sensitivity, multiplexing), better rapid test formats, integration with digital tools (software, AI/ML), automation in labs, and better data management are enhancing efficiency and reducing per-test costs. -
Regulatory Stringency & Harmonization Efforts:
As diagnostics become more critical in public health and clinical decision-making, regulatory requirements become more stringent. Regions like the EU (with IVDR), US (FDA), and others are tightening oversight, quality, validation, and documentation. Harmonization or streamlining efforts are underway but regulatory compliance remains a hurdle. -
Public Health & Screening Programs:
Governments and public health agencies are investing more in screening programs (for infectious diseases, cancer, etc.). Early detection is being prioritized; immuno diagnostics are central to many screening and monitoring strategies. -
Cost Pressures & Need for Affordable Testing:
Especially in low- and middle-income countries, or in public health settings, cost per test, supply chain logistics, access to quality reagents and instruments, and ability to scale are major concerns. There is increasing interest in cost-effective, locally manufactured immuno diagnostics.
Opportunities & Growth Drivers
Here are key opportunities that market players and investors should keep an eye on:
-
Emerging Markets Expansion: Countries in Asia-Pacific, Latin America, Middle East & Africa offer significant untapped potential. As healthcare infrastructure improves and awareness increases, demand for immuno IVD is likely to surge.
-
Innovation in Reagents & Consumables: Because reagents dominate product type segments, improvements in reagent stability, shelf-life, cost reduction, and ease of transport are major levers for growth.
-
Multiplex & Multiparameter Testing: Tests that can detect multiple markers from a single sample reduce cost, turnaround time, and patient discomfort. These are especially useful in infectious disease panels and cancer diagnostics.
-
Digital Integration & Data Management: Integration of diagnostics with data systems (cloud, AI, remote reading), telemedicine, and mobile health offers opportunities for remote diagnostics, real-time monitoring, and better epidemiological surveillance.
-
Collaborations & Strategic Partnerships: Partnerships between diagnostic firms, research institutions, governments, and NGOs can drive scale, improve regulatory navigation, and extend reach into new geographies or under-served communities.
-
Regulatory Navigation & Compliance Innovation: Firms that can efficiently achieve regulatory certification, meet quality standards, and navigate cross-border regulatory complexity will have an edge.
Challenges & Restraints
Even with strong potential, there are challenges that could slow growth or limit profitability:
-
Regulatory Hurdles: As noted, obtaining approval under different regulatory regimes is time consuming and expensive. Changes in regulation (e.g., stricter validation, new standards) can delay product launches.
-
High Cost of Instruments & Infrastructure: Instruments (especially high-throughput, automated immunoassay platforms) involve capital expense. In less developed areas, deploying such infrastructure is harder.
-
Supply Chain Constraints: Reagents, raw immunological materials, cold-chain requirements, and manufacturing consistency can be disrupted by global supply chain issues, material shortages, or geopolitical issues.
-
Competition & Price Erosion: With many players entering, competition is intensifying. This can drive price reduction, which while beneficial for consumers and public health, can squeeze margins for providers/manufacturers.
-
Quality & Reliability Concerns: False positives/negatives in diagnostics have serious clinical implications. Ensuring high specificity, sensitivity, and rigorous validation is critical, especially when deploying rapid tests or novel formats.
Strategic Recommendations for Stakeholders (2025-2032)
To capitalize on the growth in immuno IVD market, companies, public health bodies, investors, and regulators should consider:
-
Invest in R&D & Validation: Continued investment in improving sensitivity, specificity, multiplexing, and faster time to result. Clinical validation and real-world performance will be increasingly important.
-
Focus on Reagent & Consumable Efficiency: Given that reagents dominate, innovations that reduce reagent cost, increase stability, require less sample, or reduce waste will be very advantageous.
-
Localization & Market Penetration in Emerging Regions: Establish or partner for local manufacturing, adapt products to local disease profiles, make tests affordable, and meet local regulatory requirements.
-
Scale Up Point-of-Care & Rapid Testing: Build out capabilities for decentralized testing—POC, self-testing, mobile labs—especially for infectious disease, outbreak response, and remote areas.
-
Digital & Data-Driven Diagnostics Ecosystem: Leverage AI, cloud solutions, digital health, remote monitoring, telemedicine to integrate diagnostics into broader healthcare workflows.
-
Build Partnerships & Ecosystem Alliances: Collaborations with public health agencies, NGO programs, research institutions, and governments can help with outreach, regulatory support, and scaling adoption.
-
Monitor Regulatory Landscape Proactively: Be prepared for changing regulations (e.g. EU IVDR, other regional diagnostic regulation), invest in compliance, documentation, quality systems early, and streamline product registration processes.
Outlook & Market Outlook Summary
Looking ahead to 2032, the immuno IVD market is expected to continue steady growth. Key factors likely to drive next phase of expansion are:
-
Greater penetration of diagnostics in developing countries
-
More automated, multiplexed and rapid immunodiagnostic platforms
-
Increasing importance of early detection in cancer, infectious and autoimmune disease
-
Rising spending on healthcare, along with favorable policies and reimbursement systems
-
Strong innovation in reagents, assays, digital tools to reduce cost and time
By 2032, with the projected market size nearing USD 28.9 billion, the immuno IVD market will represent a critical segment of diagnostics globally, both in terms of public health impact and commercial opportunity.
Conclusion
The Immuno In-Vitro Diagnostics (IVD) market stands at a vital juncture. With a current value close to USD 19.9 billion (2024) and forecasted to reach USD 28.9 billion by 2032, the market offers strong potential for manufacturers, service providers, investors, and public health actors. Growth will be powered by rising disease burdens, demand for early detection, technological innovation, and expansion in emerging geographies.
However, success will depend on how well stakeholders can manage regulatory complexity, ensure accuracy and reliability, control costs, and scale operations to meet both urban and rural demand. Those who excel in reagent innovation, point-of-care solutions, digital integration, and geographic adaptation are likely to lead.
- Immuno_In-Vitro_Diagnostics_Market
- Immuno_IVD_Market
- Immuno_In-Vitro_Diagnostics_Market_Size
- Immuno_In-Vitro_Diagnostics_Market_Share
- Immuno_In-Vitro_Diagnostics_Market_Growth
- Immuno_In-Vitro_Diagnostics_Market_Trends
- Immuno_In-Vitro_Diagnostics_Market_Forecast_2025–2032
- Immunoassay_Market
- Diagnostic_Reagents_Market
- Point-of-Care_Diagnostics
- Rapid_Diagnostic_Testing
- Clinical_Immunodiagnostics
- Infectious_Disease_Diagnostics
- Oncology_Diagnostics_Market
- Global_Immuno_IVD_Industry
- Books
- Software
- Courses
- Películas
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Juegos
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness